In the highly regulated world of pharmaceuticals, data integrity is paramount. It forms the foundation for ensuring the safety and efficacy of medications, safeguarding public health, and maintaining trust in the industry.
What is data integrity?
Data integrity refers to the accuracy, completeness, consistency, and reliability of data throughout its lifecycle. It encompasses the entire process of data creation, capture, storage, transmission, and use.
Why is data integrity important in the pharmaceutical industry?
In the pharmaceutical industry, data plays a crucial role in various aspects, including:
- Drug discovery and development: From identifying potential drug candidates to conducting clinical trials, data is used to guide research and development decisions.
- Manufacturing: Data ensures consistent and controlled production of high-quality medications.
- Quality control: Data is used to monitor and ensure the safety and efficacy of manufactured drugs.
- Regulatory compliance: Pharmaceutical companies must comply with strict regulations, and data integrity is essential for demonstrating compliance.
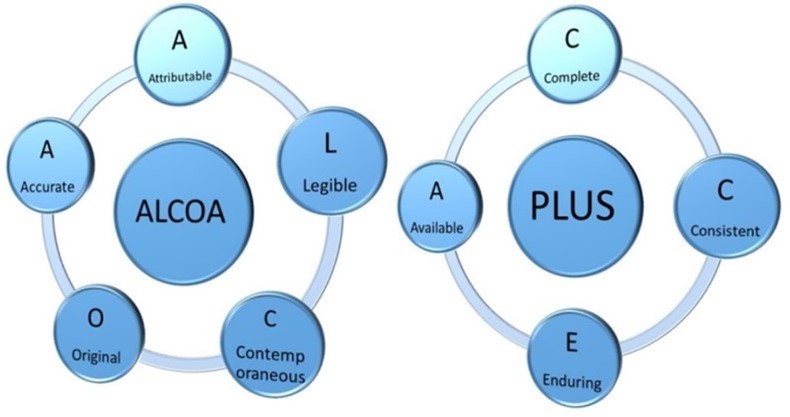
Components of data integrity:
- Attributability: Data should be clearly linked to its source, including who created it and when.
- Legibility: Data should be recorded in a clear and consistent format, using appropriate terminology.
- Contemporaneity: Data should be recorded promptly after the event or activity being documented.
- Accuracy: Data should be free from errors and omissions, reflecting the true and complete picture.
- Completeness: All relevant information should be included in the data.
- Consistency: Data should be consistent across different systems and records.
- Durability: Data should be stored in a secure and accessible manner for the required period.
- Traceability: The origin and journey of data should be demonstrably traced.
Challenges to data integrity:
Human error:
Human error is a significant challenge in the pharmaceutical industry, with estimates attributing 30-40% of all deviations to some form of human failure. These errors can have serious consequences, ranging from increased costs and production delays to product recalls, reputational damage, and even patient harm.

Understanding Human Error:
- Types of Errors:
- Slips: Unintentional actions or mistakes during routine tasks (e.g., incorrect measurements, mixing up labels).
- Lapses: Memory lapses or failures to follow procedures (e.g., forgetting a step, omitting documentation).
- Mistakes: Incorrect decisions made in good faith (e.g., misinterpreting data, choosing the wrong formula).
- Violations: Deliberate deviations from established rules or procedures (e.g., taking shortcuts, falsifying records).
- Contributing Factors:
- Work environment: Fatigue, stress, lack of sleep, distractions, poor lighting, inadequate equipment.
- Training and knowledge: Insufficient training, lack of understanding of procedures, unclear instructions, complex processes.
- Procedures and processes: Inefficient or poorly designed processes, ambiguous documentation, lack of automation.
- Organizational factors: Pressure to meet deadlines, inadequate communication, lack of safety culture, blame-oriented environment.
Addressing Human Error:
- Prevention:
- Improve training and knowledge: Provide comprehensive and regular training, ensure clear and accessible procedures, conduct regular skill assessments.
- Optimize work environment: Address fatigue and stress, improve ergonomics, minimize distractions, ensure adequate lighting and ventilation.
- Design safer processes: Automate tasks where possible, simplify procedures, use error-proofing mechanisms, double-check critical steps.
- Foster a culture of safety: Encourage open communication, report near misses without fear of blame, implement proactive safety initiatives.
- Detection and Response:
- Implement robust quality control systems: Regular inspections, audits, and monitoring to identify potential issues early.
- Thorough root cause analysis: Investigate errors to identify the underlying factors, not just the immediate cause.
- Effective corrective and preventive actions (CAPAs): Implement targeted measures to address the root causes and prevent future errors.
System issues:
Technological failures, software bugs, and inadequate controls can compromise data integrity.
Lack of awareness:
Inadequate understanding of data integrity principles and best practices can lead to unintentional errors.
Best practices for data integrity:
- Establish clear data integrity policies and procedures: Define expectations for data handling and clearly outline roles and responsibilities.
- Implement robust data management systems: Utilize technology to ensure accurate and reliable data capture, storage, and access.
- Provide training and education: Educate personnel on data integrity principles and best practices, emphasizing the importance of accurate data recording and reporting.
- Conduct regular audits and reviews: Monitor adherence to data integrity standards and identify areas for improvement.
- Promote a culture of data integrity: Foster an environment that values accurate and reliable data, encouraging transparency and accountability.
Benefits of strong data integrity:
- Increased patient safety: Ensures the quality and efficacy of medications, minimizing the risk of adverse events.
- Enhanced quality control: Facilitates effective monitoring and identification of potential product defects.
- Streamlined regulatory processes: Demonstrates compliance with regulations and facilitates smoother inspections.
- Improved decision-making: Provides accurate and reliable data for informed decisions throughout the drug lifecycle.
- Boosted public trust: Strengthens public confidence in the safety and efficacy of pharmaceuticals.
Conclusion:
Data integrity is not just about recording information; it is about upholding a culture of excellence where accuracy, completeness, and reliability are non-negotiable. In a world driven by data, integrity is not compromised, transparency is not negotiable, and reliability is not an aspiration but a standard.
Data integrity is not just a regulatory requirement; it’s a fundamental ethical obligation in the pharmaceutical industry. By implementing robust data integrity practices, pharmaceutical companies can ensure the safety and efficacy of medications, protect public health, and build trust in the industry.
- Types of Errors:



1 Comment