Good Manufacturing Practices (GMP): The backbone of Pharmaceutical Manufacturing
In the world of pharmaceuticals, there’s a crucial journey from lab breakthroughs to the pills we trust. This journey is guided by a set of strict rules called Good Manufacturing Practices (GMP). Good Manufacturing Practice (GMP) is a set of guidelines and regulations that ensure the quality, safety, and efficacy of pharmaceutical products during the manufacturing process. GMP outlines the minimum requirements for the design, construction, and operation of pharmaceutical manufacturing facilities, as well as the control and documentation of manufacturing processes. These rules are the backbone of the pharmaceutical manufcaturing. Let’s dig into what GMP is all about – its key components, why it matters, the pharmaceutical GMP guidelines, the benefits of GMP, and where it’s headed in the future.
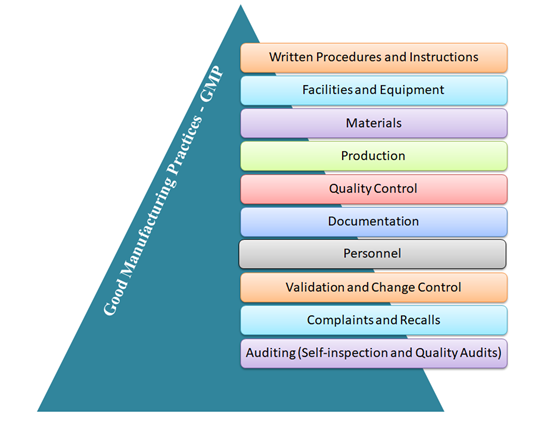
Key Components of GMP:
Written Procedures and Instructions
Detailed, written procedures are essential for each process that could affect the quality of the finished product. There must be systems to provide documented proof that correct procedures are consistently followed at each step in the manufacturing process.
Facilities and Equipment
Facilities and equipment should be properly designed, maintained, and cleaned to ensure the quality of products. Equipment validation and calibration are also crucial for maintaining consistent operations.
Materials
All materials used in a manufacturing process must be of tested quality, clearly identified, and readily traceable. This includes both the product’s ingredients and the specific containers and closures that will be used.
Production
Manufacturing processes are clearly defined, controlled, and validated to ensure consistency and compliance. Critical processes are validated to demonstrate that they are capable of consistently delivering quality products.
Quality Control
Products must be tested at different stages of production to verify quality. These tests should ensure the identity, purity, potency, and quality of the product.
Documentation
Records, raw data, and documents related to production and distribution should be retained and available for review. They should be clear, comprehensive, and accurate.
Personnel
Qualified and adequately trained personnel are essential. Every person involved in manufacturing should have the education, training, and experience to perform their role effectively.
Validation and Change Control
Changes to the manufacturing process must be properly reviewed, validated, and documented to ensure the quality of the product isn’t compromised.
Complaints and Recalls
There should be systems in place for handling complaints and product recalls. This involves reviewing and investigating complaints and taking appropriate corrective actions when necessary.
Auditing (Self–inspection and Quality Audits)
Regular audits should be conducted to ensure that GMP guidelines are being followed. These audits can identify areas for improvement and ensure that corrective actions are implemented.
Current Good Manufacturing Practice (cGMP)
Current Good Manufacturing Practice, also known as cGMP, is a set of regulations that ensure the quality of pharmaceutical products, medical devices, biotechnology products, food and beverage, and dietary supplements. They therefore apply to all organizations involved in the manufacturing processes of these products.
To be compliant with the cGMP regulations, companies must meet the latest quality standards. These apply to buildings and facilities, equipment, production, process controls, laboratory controls, packaging and labeling, and returned or salvaged drug products.
Current Good Manufacturing Practice (cGMP) is an evolved form of GMP, emphasizing contemporary standards and practices in pharmaceutical manufacturing. It encompasses the application of modern technologies, risk management approaches, and a focus on continuous improvement throughout the product life cycle.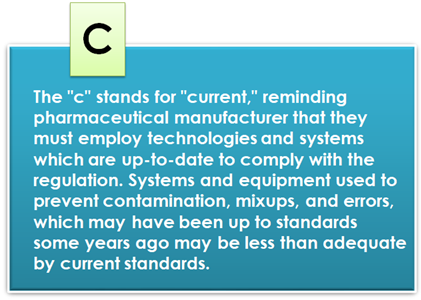
Enhanced Features of cGMP:
Risk-Based Approaches
Incorporates risk assessment methodologies into manufacturing processes.
Process Validation
Emphasizes the need for ongoing process validation to ensure consistent product quality.
Quality Risk Management
Integrates a comprehensive risk management framework to identify, assess, and control risks to product quality.
GMP Guidelines
Several pharmaceutical Good Manufacturing Practice (GMP) guidelines exist worldwide, each aimed at ensuring the quality, safety, and efficacy of pharmaceutical products. Here are some of the key GMP guidelines from different regions:
- United States:21 CFR Part 210 and 211: These regulations by the U.S. Food and Drug Administration (FDA) outline CGMP requirements for finished pharmaceuticals.
- ICH Q7: The International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) developed this guideline to establish GMP requirements for active pharmaceutical ingredients (APIs).
- FDA Guidance Documents: The FDA publishes numerous guidance documents to provide additional clarification and recommendations on various aspects of pharmaceutical GMP.
- European Union:EU GMP Guidelines: The European Union has its own set of GMP guidelines, which cover various aspects of pharmaceutical manufacturing and quality control. These guidelines are detailed in Volume 4 of the EU Guidelines to Good Manufacturing Practice.
Annex 1: This EU GMP guideline specifically addresses the manufacture of sterile medicinal products, providing requirements for facilities, equipment, personnel, and processes. - International:
ICH Guidelines: The International Council for Harmonisation has developed several guidelines covering various aspects of pharmaceutical development, quality, safety, and efficacy. These include guidelines such as ICH Q8 (Pharmaceutical Development), ICH Q9 (Quality Risk Management), and ICH Q10 (Pharmaceutical Quality System).
WHO GMP: The World Health Organization (WHO) publishes guidelines on GMP for pharmaceutical products, including recommendations for the production, control, storage, and distribution of pharmaceuticals. - Japan:
JP GMP: Japan’s Pharmaceutical and Medical Devices Agency (PMDA) provides GMP guidelines known as “J-GMP” or “JP GMP.” These guidelines align with international standards while addressing specific regulatory requirements in Japan. - Canada:
Health Canada GMP Guidelines: Health Canada provides GMP guidelines for pharmaceutical products to ensure compliance with Canadian regulatory requirements. - Australia:
PIC/S GMP Guide: Australia adopts the Pharmaceutical Inspection Co-operation Scheme (PIC/S) GMP Guide, which provides internationally harmonized GMP standards for pharmaceutical manufacturing. - International Pharmaceutical Regulators Forum (IPRF):
GMP Guidelines and Recommendations: The IPRF comprises pharmaceutical regulatory authorities from various countries and regions. It collaborates to develop and harmonize GMP guidelines and recommendations to ensure consistent standards for pharmaceutical manufacturing worldwide. Compliance with these GMP guidelines is essential for pharmaceutical manufacturers to meet regulatory requirements and ensure the quality, safety, and efficacy of their products. Pharmaceutical companies must stay updated with evolving guidelines and standards to maintain compliance and protect public health.
Benefits of GMP Compliance
Increased Patient Safety
By upholding the principles of GMP, the pharmaceutical industry minimizes the risk of adverse events, ensuring that every medication reaching patients is of the highest standard of safety.
Enhanced Product Efficacy
GMP practices guarantee that drugs are manufactured to the intended specifications, maximizing their effectiveness and therapeutic impact.
Reduced Manufacturing Costs
Implementing efficient and controlled manufacturing processes not only ensures quality but can also lead to cost savings, contributing to the sustainability of the pharmaceutical industry.
Improved Public Trust
Adherence to GMP standards fosters public trust in the quality and safety of pharmaceutical products. Patients can confidently rely on medications knowing they are produced under stringent guidelines.
Future of GMP: Evolution in the Pursuit of Quality
Harmonization of Standards
Efforts are underway to harmonize GMP standards internationally, simplifying compliance for global manufacturers. This unified approach aims to streamline processes and ensure consistent quality across borders.
Technological Advancements
The future of GMP will likely witness the integration of new technologies, such as automation and data analytics, to improve compliance and efficiency in pharmaceutical manufacturing. These tools offer real-time insights and enhance the overall manufacturing process.
To Sum up
In conclusion, Good Manufacturing Practices stand as the guardians of pharmaceutical excellence, ensuring that every pill, injection, or vial meets the highest standards of quality, safety, and efficacy. The commitment to GMP is a commitment to patients, a promise that the pharmaceutical industry upholds as it face the complexities of drug development, manufacturing, and distribution.
As the pharmaceutical world keeps changing, GMP will also grow and adapt. The future looks bright, with a pharmaceutical industry where people everywhere can rely on getting top-notch medications every time they need them. This sets the stage for a world where good health isn’t just a hope but something everyone can count on.In the journey of pharmaceutical excellence, GMP plays a pivotal role, harmonizing the elements of quality, safety, and efficacy into a composition that resonates across borders, reaching patients and communities with the assurance of pharmaceutical excellence.


